Hello everyone, today in Chemistry Mrs. Mandarino stamped our homework. Once again we did not have a chance to switch seats because we went straight to our lab. First Mrs. Mandarino explained the titration 3 lab and told us how to do everything. We went to go do the lab and it did not go smoothly for our group. The purpose of this lab was to apply titration to determine the precise molarity of an NaOH solution using a known solid acid. After we found all of our data we recorded it and started the post lab questions but we ran out of time. Our homework was to finish the lab.
-Simone
Chemistry 163 Period 5 Scribe Post Blog
Friday, April 15, 2011
Thursday, April 14, 2011
Titration Station
Hello friends and family, it's time for the blog you've all been waiting for. There isn't a Cubs game tonight so I can do my homework.
Before I teach you how to dougie, I'm going to teach how to do Titration. Ok here's some examples of the problems we have been doing in class over the pass couple of days.
MaVa=MbVb
Acids and Bases my friends
Ph 0-14 1-6 acid 7 neutral 8-14 base
First, complete and balance your equation.
HBr + Ca(OH)2 -------> CaBr2 + 2H2O
A 50 mL of HBr is titrated to an end point with 24 mL of 1.5 M NaOH. What is the concentration of the HBr?
Ma(50mL)=(1.5M)(24mL)
Ma= .72M
Let's take it to the next level shall we?
First here's some notes I took.
1 x 10^-5 Predicted Ph=5 Acid
pH=6 pOH= 8
pH= 3 pOH=11
Alright so I hope that helps everyone, the homework was to finish the box on page 4. Also on page 5 do all the problems except 2 d. and 3 a.
Μαντώ, είστε τα γόνατα μελισσών. Μακάρι να μπορούσαμε να γίνουμε φίλοι και πάλι,
συγγνώμη για όλα λάθος κατορθώματα μου. Χαιρετισμοί
συγγνώμη για όλα λάθος κατορθώματα μου. Χαιρετισμοί
Tuesday, February 22, 2011
Molarity
The Agenda
4.) H2CO3(aq) + 2KOH(aq) ---> 2H2O + K2CO3(s)
5.) CdBr2(aq) + Na2S(aq) ---> CdS(s) + 2NaBr(aq)
6.) 2NaOH(aq) +Ba(NO3)2(aq) ---> 2NaNO3(aq) + Ba(OH)2(aq)
Molarity
M =moles of solute
liters of solution
Its like stoich but there's Liters in it. There are some stuff that Mrs. Mandarino has not covered yet,
she just talked about the equation of Molarity and answered some of the questions in Molarity sheet 1 and 2
Molarity sheet 1
1.)What is the molar mass of the following compounds? Write dissociation reactions for each of them.
(so basically, you just have to find the molar mass- easy)
a.) NaNO3 (1 x 14.01g) + (1 x 22.99g) + (3 x 16.00g) = 85g
b.) CaCl2 (1 x 40.08) + (2 x 35.45g) = 110.48g
2.)Calculate the number of moles of the following (yeah, step by step)
a.) 25g of NaNO3 x 1mol NaNO3 = 294 mol NaNO3
85gNaNO3
b.) 200g of CaCl2 x 1mol CaCl2 =1.8 mol CaCl2
110.98g CaCl2
3.) Calculate the molarity of the following solutions. (You will use the equation in here- new stuff)
a.) 0.25 liters of solution contains 0.0346 moles of NaNO3
M= 0.0346 mol = .1384 mol/L or .138 M
0.250 L
b.) 7.54 liters of solution that contains 0.75 moles of CaCl2
M= 0.75 mol = 15 M
7.54 L
c.) 100.0 ml of solution that contains 1.5 moles of C6H12O6 (Pro tip: watch out for the measurements, we
need liters not milliliters, so it's .1L of solution. Also C6H12O6 is Glucose)
M= 1.5 mol = 15 M
.1 L
d.) 560 ml (.56L) of solution that contains 0.025 moles of AgNO3
M= .02 mol = .036 M
.56 L
Molarity sheet 2
4.) Calculate the molarity of the following solutions.
a.) 1.5 liters of a solution that contains 55.5 g of NaNO3
55.5g NaNO3 x 1 mol NaNO3 = .653 mol NaNO3 (because we want the = .44 M
85.0g NaNO3 1.50L molarity of the solution)
5.) Calculate the moles of solute in the following solution.
a.) 0.2 liters of 0.125 M C6H12O6
.2 L x .125 mol = .025 mol C6H12O6
1L
6.) Calculate the volume of the following solutions.
a.) 0.5 moles of CaCl2 makes of a 0.667 M solution
0.5 mol x L =.750 L CaCl2
.667mol
Well that's all folks. Remember to finish the other questions in Molarity sheet 1 and 2 for homework tomorrow. Furthermore, the webassign are posted and ready to be answered.
- Stamp the homework (4-6 in Reaction solubility and the lab)
- Check homework
- Start Molarity
4.) H2CO3(aq) + 2KOH(aq) ---> 2H2O + K2CO3(s)
5.) CdBr2(aq) + Na2S(aq) ---> CdS(s) + 2NaBr(aq)
6.) 2NaOH(aq) +Ba(NO3)2(aq) ---> 2NaNO3(aq) + Ba(OH)2(aq)
Molarity
M =moles of solute
liters of solution
Its like stoich but there's Liters in it. There are some stuff that Mrs. Mandarino has not covered yet,
she just talked about the equation of Molarity and answered some of the questions in Molarity sheet 1 and 2
Molarity sheet 1
1.)What is the molar mass of the following compounds? Write dissociation reactions for each of them.
(so basically, you just have to find the molar mass- easy)
a.) NaNO3 (1 x 14.01g) + (1 x 22.99g) + (3 x 16.00g) = 85g
b.) CaCl2 (1 x 40.08) + (2 x 35.45g) = 110.48g
2.)Calculate the number of moles of the following (yeah, step by step)
a.) 25g of NaNO3 x 1mol NaNO3 = 294 mol NaNO3
85gNaNO3
b.) 200g of CaCl2 x 1mol CaCl2 =1.8 mol CaCl2
110.98g CaCl2
3.) Calculate the molarity of the following solutions. (You will use the equation in here- new stuff)
a.) 0.25 liters of solution contains 0.0346 moles of NaNO3
M= 0.0346 mol = .1384 mol/L or .138 M
0.250 L
b.) 7.54 liters of solution that contains 0.75 moles of CaCl2
M= 0.75 mol = 15 M
7.54 L
c.) 100.0 ml of solution that contains 1.5 moles of C6H12O6 (Pro tip: watch out for the measurements, we
need liters not milliliters, so it's .1L of solution. Also C6H12O6 is Glucose)
M= 1.5 mol = 15 M
.1 L
d.) 560 ml (.56L) of solution that contains 0.025 moles of AgNO3
M= .02 mol = .036 M
.56 L
Molarity sheet 2
4.) Calculate the molarity of the following solutions.
a.) 1.5 liters of a solution that contains 55.5 g of NaNO3
55.5g NaNO3 x 1 mol NaNO3 = .653 mol NaNO3 (because we want the = .44 M
85.0g NaNO3 1.50L molarity of the solution)
5.) Calculate the moles of solute in the following solution.
a.) 0.2 liters of 0.125 M C6H12O6
.2 L x .125 mol = .025 mol C6H12O6
1L
6.) Calculate the volume of the following solutions.
a.) 0.5 moles of CaCl2 makes of a 0.667 M solution
0.5 mol x L =.750 L CaCl2
.667mol
Well that's all folks. Remember to finish the other questions in Molarity sheet 1 and 2 for homework tomorrow. Furthermore, the webassign are posted and ready to be answered.
Friday, February 18, 2011
Solutions
Since Dan has failed us twice, I am sharing 8th period's scribe:
Yesterday in class we finished our Mixtures Lab. We took each of the four mixtures and completed the evaporation test and went on to the filtrate lab. To fill out the back sheet of the paper, we can use the notes we took in class on Wednesday. If you want to check if you got the correct results, they are on moodle.
We also learned about solubility and dissolving. We learned that there are two categories of solutions: the solutes and the solvents.
Solutes:
-What is being dissolved
-Usually smaller amount
Solvents:
-What deos the dissolving
-Usually the greater amount
Ionic Compounds: When they are dissolved in water they split into ions (dissociation)
Covalent Compounds: When they are dissolved in water they do not split
*When the compounds are dissolved in water you change the subscrips to (aq), which means in water*
The homework is to finish the Mixtures Lab.
[The notes Mrs. Mandarino gave us in class are on moodle, in the notes section.
If you missed ChemDay, the make up assignment can be found in the homework log section on moodle.]
Yesterday in class we finished our Mixtures Lab. We took each of the four mixtures and completed the evaporation test and went on to the filtrate lab. To fill out the back sheet of the paper, we can use the notes we took in class on Wednesday. If you want to check if you got the correct results, they are on moodle.
We also learned about solubility and dissolving. We learned that there are two categories of solutions: the solutes and the solvents.
Solutes:
-What is being dissolved
-Usually smaller amount
Solvents:
-What deos the dissolving
-Usually the greater amount
Ionic Compounds: When they are dissolved in water they split into ions (dissociation)
Covalent Compounds: When they are dissolved in water they do not split
*When the compounds are dissolved in water you change the subscrips to (aq), which means in water*
The homework is to finish the Mixtures Lab.
[The notes Mrs. Mandarino gave us in class are on moodle, in the notes section.
If you missed ChemDay, the make up assignment can be found in the homework log section on moodle.]
Tuesday, February 8, 2011
Limiting Reactants
Limiting Reactant Strategy
If a problem mentions amounts of more than one reactant, its probably a limiting reactant problem.
For Example:
Fe + S à FeS
If a problem mentions amounts of more than one reactant, its probably a limiting reactant problem.
- Go from each reactant to amount of product (grams of each reactant to grams of product)
- The reactant that produces the lower amount of predicted product is the limiting reactant. This amount of product is also the amount that can be produced.
- The other reactant is your excess reactant.
For Example:
Fe + S à FeS
If I have 28g of Iron (56 g/mol) and 24g of Sulfur (32 g/mol), how much FeS can be made and what is the limiting reactant?
28 g Fe x 1 mol Fe x 1 mol FeS x 87.92g FeS = 44 g FeS
55.85 g Fe 1 mol Fe 1 mol FeS
24 g S x 1 mol S x 1 mol FeS x 87.92g FeS = 66 g FeS
32.07 g S 1 mol S 1 mol FeS
Fe is the Limiting reactant because it runs out first and produces less FeS.
Tuesday, February 1, 2011
Stoichiometry
Today in Mando's fifth period chem class we did a multitude of things. we went over the homework which was pages fifteen and sixteen. After that we spent the rest of the class taking our Stoich quiz, you must score a one-hundred percent on at least one of the Stoich quizes to take the unit test. the homework for tonight is pages 17 and 18.
Also see the cheat sheet in the Unit 8 Stoich notes on Moodle!!!
Jack H.
Also see the cheat sheet in the Unit 8 Stoich notes on Moodle!!!
Jack H.
Tuesday, January 25, 2011
Moles and Molar Mass
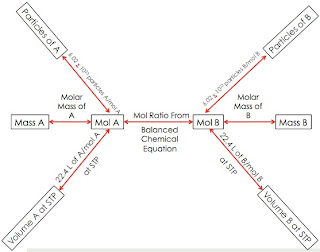
Today in chemistry, we picked up additional worksheets that went along with Unit 8. We then worked on pages 7 and 8 to refresh our minds on ionic and covalent formulas. Remember that an ionic compound consists of a metal and a non-metal. Covalent compounds consists of only non-metals. Writing the name of the formula depends on what compound it is. On page 8 of the review, we started to determine the molecular mass of compounds. When given a compound, you must write out the formula first. Then, count how many atoms are in the first element and multiply that number by the element's atomic mass. The atomic mass can be found on the periodic table. Do the same thing with the second element in the formula. Then, add these two products together and the sum you get is the molar mass. An example would be like this:
Compound given: carbon dioxide
Formula: CO2
There is one C so you multiply 1 by the atomic mass. Carbon's atomic mass is 12.01 grams. 1 x 12.01 g. Looking at oxygen, it has two atoms. The atomic mass of Oxygen is 16.00 grams. 2 x 16.00 g. Then you add these two products. The answer is 44.01. The equation should look like this: (1 x 12.01 g) + (2 x 16.00 g) = 44.01 grams.
The next thing we did was how to calculate the number of moles. You had to apply your knowledge of molar mass to figure out how to do this. It is like unit conversion. An example would be like this: 50 g of carbon dioxide.
You should write down the number that is given to you so you should write out 50 g of CO2. You need to multiply this by something. Since we want to figure out the number of moles in a compound, we would write 1 mole of CO2 at the top of the dividing line. At the bottom would be the molar mass of CO2 and the unit should be g of CO2 since we want to cancel this out. You then calculate all this and the answer should come out to be 1.14 moles of CO2. The equation should look like this:
50 g of CO2 x 1 mole of CO2/44.01 g of CO2 = 1.14 moles of CO2.
After completing pages 7 and 8, we started on page 1. It pretty much had the same concept on what we did on page 8. Near the end of class, Mrs. Mandarino stamped the labs.
Homework is to complete worksheets pg 1 and 2!
Compound given: carbon dioxide
Formula: CO2
There is one C so you multiply 1 by the atomic mass. Carbon's atomic mass is 12.01 grams. 1 x 12.01 g. Looking at oxygen, it has two atoms. The atomic mass of Oxygen is 16.00 grams. 2 x 16.00 g. Then you add these two products. The answer is 44.01. The equation should look like this: (1 x 12.01 g) + (2 x 16.00 g) = 44.01 grams.
The next thing we did was how to calculate the number of moles. You had to apply your knowledge of molar mass to figure out how to do this. It is like unit conversion. An example would be like this: 50 g of carbon dioxide.
You should write down the number that is given to you so you should write out 50 g of CO2. You need to multiply this by something. Since we want to figure out the number of moles in a compound, we would write 1 mole of CO2 at the top of the dividing line. At the bottom would be the molar mass of CO2 and the unit should be g of CO2 since we want to cancel this out. You then calculate all this and the answer should come out to be 1.14 moles of CO2. The equation should look like this:
50 g of CO2 x 1 mole of CO2/44.01 g of CO2 = 1.14 moles of CO2.
After completing pages 7 and 8, we started on page 1. It pretty much had the same concept on what we did on page 8. Near the end of class, Mrs. Mandarino stamped the labs.
Homework is to complete worksheets pg 1 and 2!
Subscribe to:
Comments (Atom)